During this school holidays, there is this programme called Autumn Learning Journey. It is where you complete as many activities as you can. One of the activities is making an Autumn garden in Minecraft. I did my Autumn garden on a flat world.
First of all, I created the boundary. Then, I dug up some of the grass to make a path. I accidentally made one side bigger than the other. On the big side, I mine the grass and refilled it with water. On the other side, I ended up having nothing in it. I was first thinking of planting wheat but I found out that it was impossible on a flat world. So I place podzol around the water and planted mushrooms instead.
I made a tree with the leaves red, orange and yellow to represent Autumn. I place pumpkins around the trunk. I made a pen and spawned a fox named Autumn. I used a fox because google showed up the a fox is and Autumn animal. I spawned heaps of chickens to feed the fox, accidentally let the chickens out, spawned heaps of other animals to even it out. I spawned more foxes outside and lots more chickens in the pen. After a while, more foxes joined Autumn and I ended up feed Autumn and whole bunch of her friends. I spawned a bit too much animals so the world is a bit glitchy.
I am riding a horse to take my screenshots because it gets me a better height to adjust and capture.
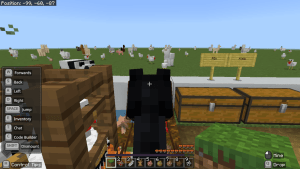
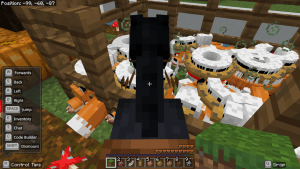
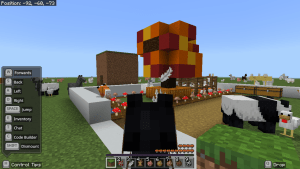
What would you put in an Autumn garden?